Modelling the Mechanobiology of Valve Cells
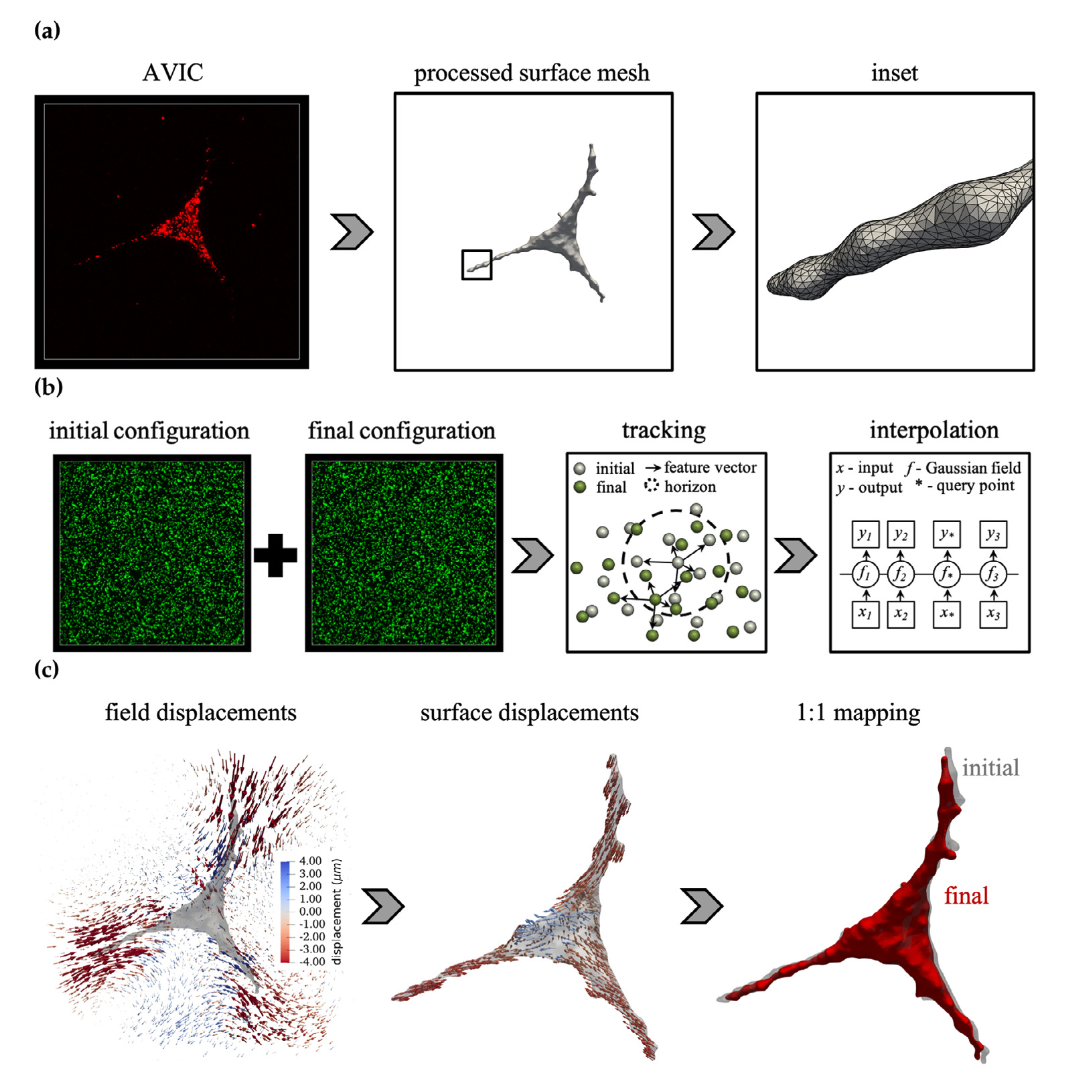
Valve interstitial cells (VICs) reside within the cardiac valves and primarily adopt a quiescent phenotype under homeostatic conditions. Altered biochemical and mechanical microenvironments can stimulate VICs to undergo a myofibroblast phenotypic activation characterized by increases in extracellular matrix synthesis and remodeling as well as an increase in cell contractility. This VIC activation allows heart valves to adapt with the organism in growth/developmental processes but is also implicated in virtually all valve diseases. This project seeks to develop a computational model of the AVIC, bridging the cell-level mechanical function to the subcellular-level signaling events that give rise to AVIC activation. Ultimately, these models will serve as an in silico drug target identification platform for the development of novel, non-surgical interventions for valvular heart disease.
The three-dimensional shapes and contractile behaviors of AVICs are assessed within tissue-mimicking synthetic poly(ethylene glycol) hydrogels using standard 3D traction force microscopy techniques. AVIC membranes are stained and imaged in full 3D, along with green, fluorescent markers which allow for the quantification of the hydrogel deformation in response to AVIC-induced contraction. From this data, we developed the first 3D contractile computational model of the AVIC, which has provided unprecedented insight into how AVICs strengthen their microenvironment through deposition of extracellular matrix components. In addition, it has allowed for the prediction of underlying AVIC stress fiber architecture and contractile forces in healthy and diseased scenarios.