Research Projects
Patient Specific Quantification and Simulation of Mitral Valve Function

Dr. Amir Khalighi

Bruno Rego

Harshita Narang
The mitral valve (MV) plays an integral role in regulating blood flow throughout the cardiac cycle, and the assessment of MV function is important in many diagnostic, prognostic, and surgical planning applications. Recently, we have developed a novel noninvasive computational method to estimate MV leaflet membrane strains from clinically obtained real-time three-dimensional echocardiography (rt-3DE) images, which are segmented to produce meshed geometries of the open-state and closed-state leaflet medial surface. An image-based morphing pipeline was implemented within a finite element (FE) modeling framework, in which MV closure was simulated by pressurizing the open-state geometry, and local corrective loads were automatically applied to enforce the overall imaged closed shape of the valve. A complete map of local systolic strains was then obtained from the final FE mesh configuration. Our method estimated local strains with less than 10% error and proved to be robust against changes in boundary conditions similar to those observed in disease conditions, suggesting that this approach can be used to accurately assess the deformation state of patient-specific MVs in both normal and certain perturbed conditions.

Modeling and simulation of the normal, diseased, and replacement aortic heart valve

Dr. Rana Zakerzadeh

Sam Potter

Wenbo Zhang
Undergraduate Assistant
Jacob Sansom
Nicholas West
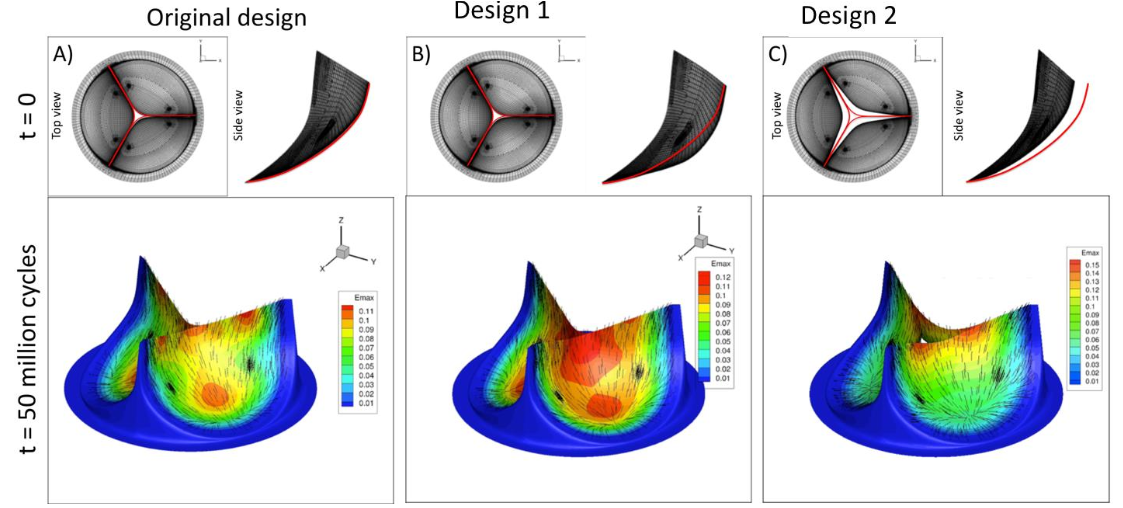
Development of an integrated approach to facilitate BHV biomaterial fabrication and predicting its in vivo performance under realistic stress levels in blood contact. The project is done in collaboration with Penn State and Iowa Sate, utilizing industry standard accelerating test protocols to evaluate the feasibility and time evolving biomechanics of the biomaterial under long-term cyclic loading conditions in vitro, as well as rationally developed constitutive models and rigorous computational implementations.
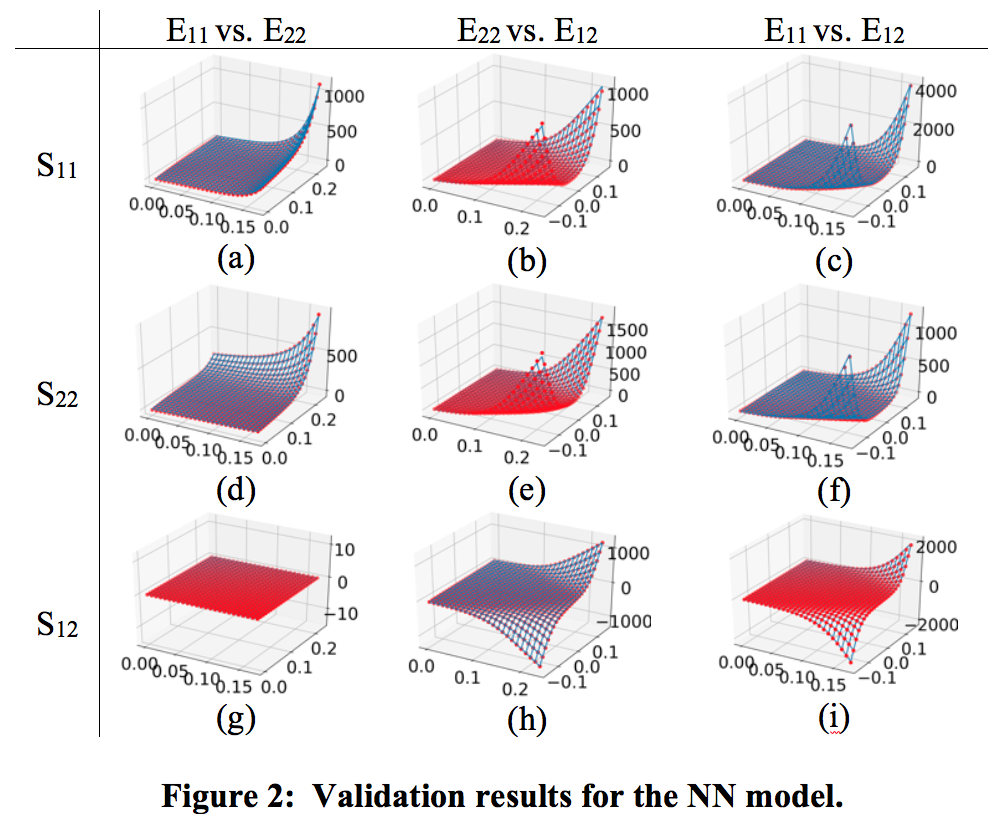
Wenbo's work involves complex mechanical behaviors, where the high-fidelity mesoscale/multiscale methods for soft tissue models are attractive and predictive but mostly computationally expensive. Traditional phenomenological models often have simple forms based on physical insights, but they lack ability to account for complex material behaviors. For modeling and remodeling of the bioprosthetic heart valve (BHV), predictive structural constitutive models have been developed for time independent and time evolving properties of exogenously crosslinked collagenous soft tissues under cyclic loading. To simulate BHV designs or further identify mechanical properties, an efficient constitutive model is crucial. Neural networks have been attracting more attentions because of its high representation capability and flexible designs. To this end, we investigate possible approaches to build a neural network (NN) model that can replicate the responses of the full structural model with traceable computational cost. The fact that the NN model is trained on the data generated by the structural model instead of experimental data would implicitly impose some proper regularization to let the fitting problem more well-defined.

Heart Valve Interstitial Cell Mechanobiology

Dr. Daniel P. Howsmon

Dr. Emma Lejeune

Alex Khang
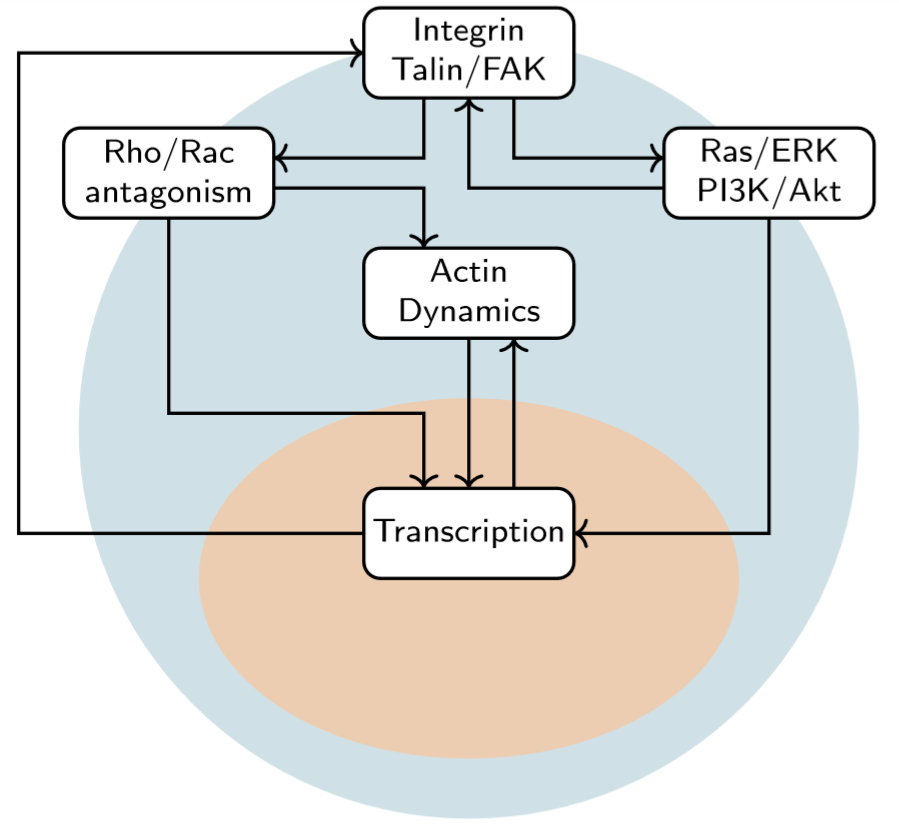
Valve interstitial cells (VICs) are the primary cells inhabiting the cardiac valves and they primarily adopt a quiescent phenotype under homeostatic conditions. Altered biochemical and mechanical microenvironments can stimulate VICs to adopt an activated phenotype characterized by increased contractions and extracellular matrix synthesis and remodeling. This VIC activation allows heart valves to adapt with the organism to growth/development processes but is also thought to underlie many valve diseases. This project seeks to develop a computational model of the signaling mechanisms responsible for VIC activation and eventually serve as an in silico drug target identification platform for the development of pharmaceutical interventions for combatting valve diseases.
Alex's work involves the Aortic valve interstitial cells (AVIC), which are fibroblast-like cells that exert tension on their immediate environment as a sensing mechanism to probe the local mechanics of the aortic valve’s extracellular matrix (ECM). These traction forces produced by the AVICs play a regulatory role towards the biosythensis of ECM components. We seek to quantify the traction forces exerted by AVICs within a 3D hydrogel environment. Fluorescent microbeads are embedded within a PEG hydrogel and are used to track the deformation resulting from AVIC contraction. The AVICs are stained and imaged to extract the cell geometry and asses the volumetric changes associated with contraction. From this, we will develop a full 3D finite element model of the AVIC.

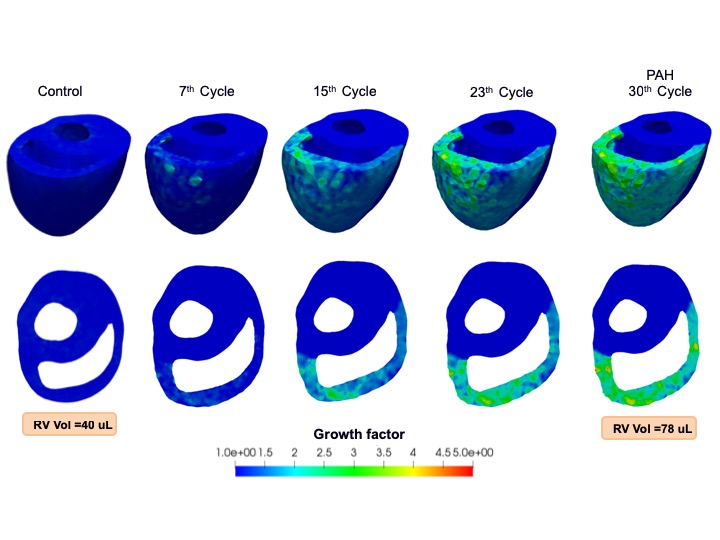
Cardiac Remodeling Under Pulmonary Hypertension

Dr. Reza Avazmohammadi
Undergraduate Assistant
Emilio Mendiola
The hypertrophy and remodeling of the heart right ventricle (RV) are key predictors of right heart failure in patients with pulmonary arterial hypertension (PAH) disease. These effects are induced by pressure overload in the RV and are associated with structural and mechanical changes of the RV free wall (RVFW) that eventually results in RV failure. New tools are needed to allow clinicians to use in vivo measurements of RV function into a model that can help predict the progression of this disease. Our long-term objective for this project is to improve our understanding on how the RV adapts to PAH, and to develop an experimentally-guided growth and remodeling (G&R) model that captures this adaptation. This model can be “personalized” using in vivo imaging data and thereby has the potential to provide the means for clinicians to predict the progression of RV hypertrophy and evaluate the efficacy of new clinical interventions.
Effects of Myocardial Infarction

David Li

Hao Liu
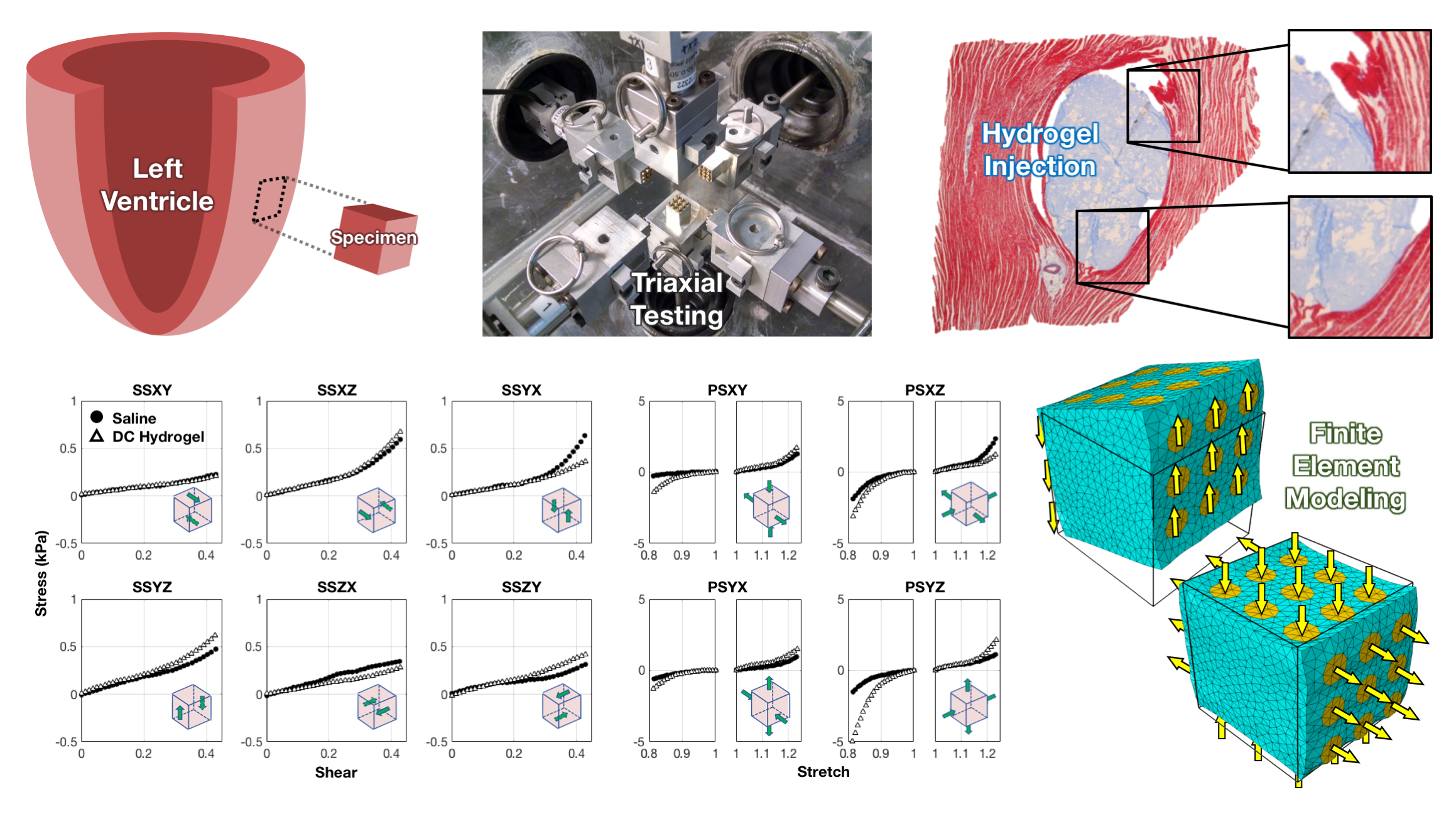
Recent studies have demonstrated that detrimental remodeling of the left ventricle after myocardial infarction (heart attack) can be mitigated through the injection of biomaterials to reinforce the affected tissue. Optimization of these therapies is still limited by a lack of biomechanical knowledge of the local and regional mechanical events that occur as a result of the presence of the biomaterial; however, their application can be systematically improved through simulations that incorporate 3D local mechanics. To this end, we have implemented numerical-experimental methodologies to obtain structural-mechanical measurements from ovine myocardium in full 3D in states of health, disease, and treatment (specifically hydrogel injection). Our approach integrates optimal design of experiments, novel mechanical experimentation and imaging, and finite element modeling to characterize the tissue-level properties of myocardium with robust constitutive models.