Research Projects
Mitral Valve Modeling and Mechanobiology
Dr. Chung-Hao Lee and Salma Ayoub
The development of a high fidelity and micro-anatomically accurate computational model for heart mitral valves with
applications to the patient-specific modeling. Specific topics include image segmentation of high-resolution MicroCT
and/or patient-specific ultrasound data, reconstruction of 3D micro-structurally accurate mitral valve geometry,
mapping of collagen fiber architecture onto mitral valve model, and finite element simulations of mitral valve
closure.

Mitral Valve Geometry Characterization and Development of an MV Population-Averaged Model
Dr. Andrew Drach and Amir Khalighi
Simulations of the biomechanical behavior of the Mitral Valve (MV) based on simplified geometric models are
difficult to interpret due to significant intra-patient variations and pathologies in the MV geometry. Thus, it is
critical to use a systematic approach for characterizing the MV and population-averaging the patient-specific
models. We are working on a multi-scale modeling framework for characterizing the entire MV apparatus geometry. MV
is comprised of morphologically distinct parts (the planar topology of leaflets vs. tubular structure of chordae
tendineae) and that requires specific treatment for each part. The methodology is based on the analysis of
high-resolution imaging data of MV and enables us to describe the entire MV geometry with a relatively small set of
parameters. Statistical analysis is then performed to develop the average MV model.
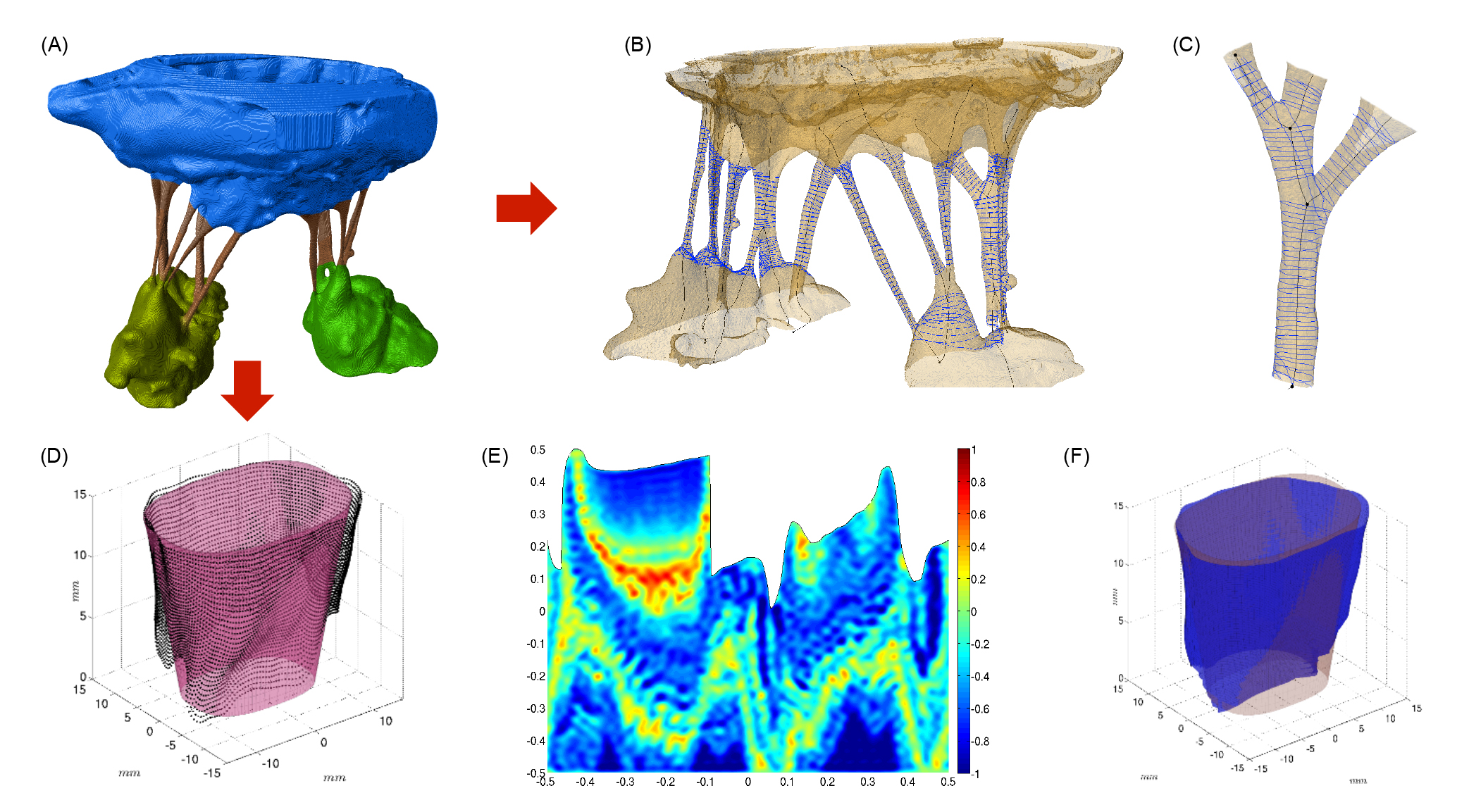
Figure 1. (A) CT data, leaflets are shown as blue, chordae tendineae are shown as red, and green represents
papillary muscles. (B) Chordal structure. (C) Branching in chordal structure. (D) Point cloud data is shown as
black, and the large-scale model is shown as a shaded surface. (E) Unfolded view of a reconstructed fine-scale
model, lower boundary corresponds to annulus, upper boundary is free edge, colormap represents normalized deviations
from the fitted surface. (F) Reconstructed surface using the two-scale model.
Simulation & Quantification of MVIC Deformations Under Physiological Loading
Dr. Chung-Hao Lee and Salma Ayoub
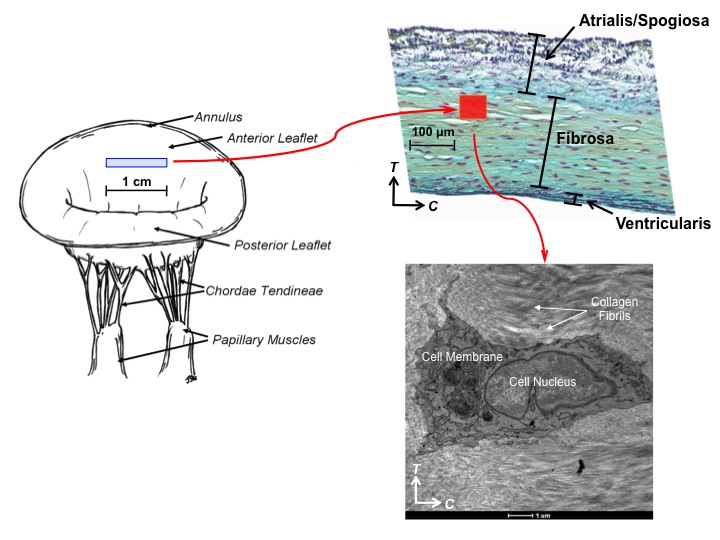
Within each of the four layers of mitral valve (MV) leaflets there resides a heterogeneous population of
interstitial cells that maintain the structural integrity of the MV tissue via protein biosynthesis and enzymatic
degradation. There is increasing evidence that tissue stress-induced MV interstitial cell (MVIC) deformations can
have deleterious effects on their biosynthetic states that are potentially related to the reduction of tissue-level
maintenance and to subsequent organ-level failure.
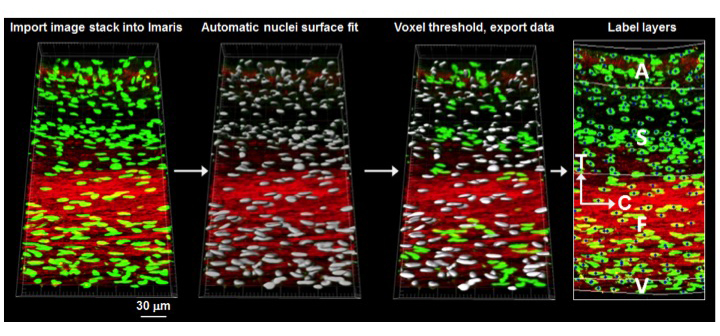
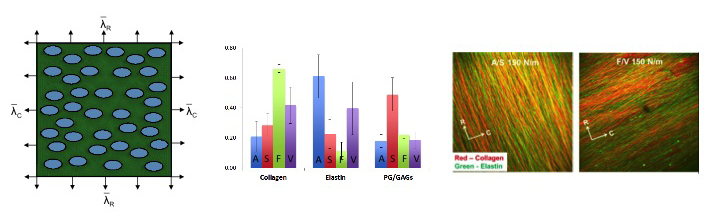
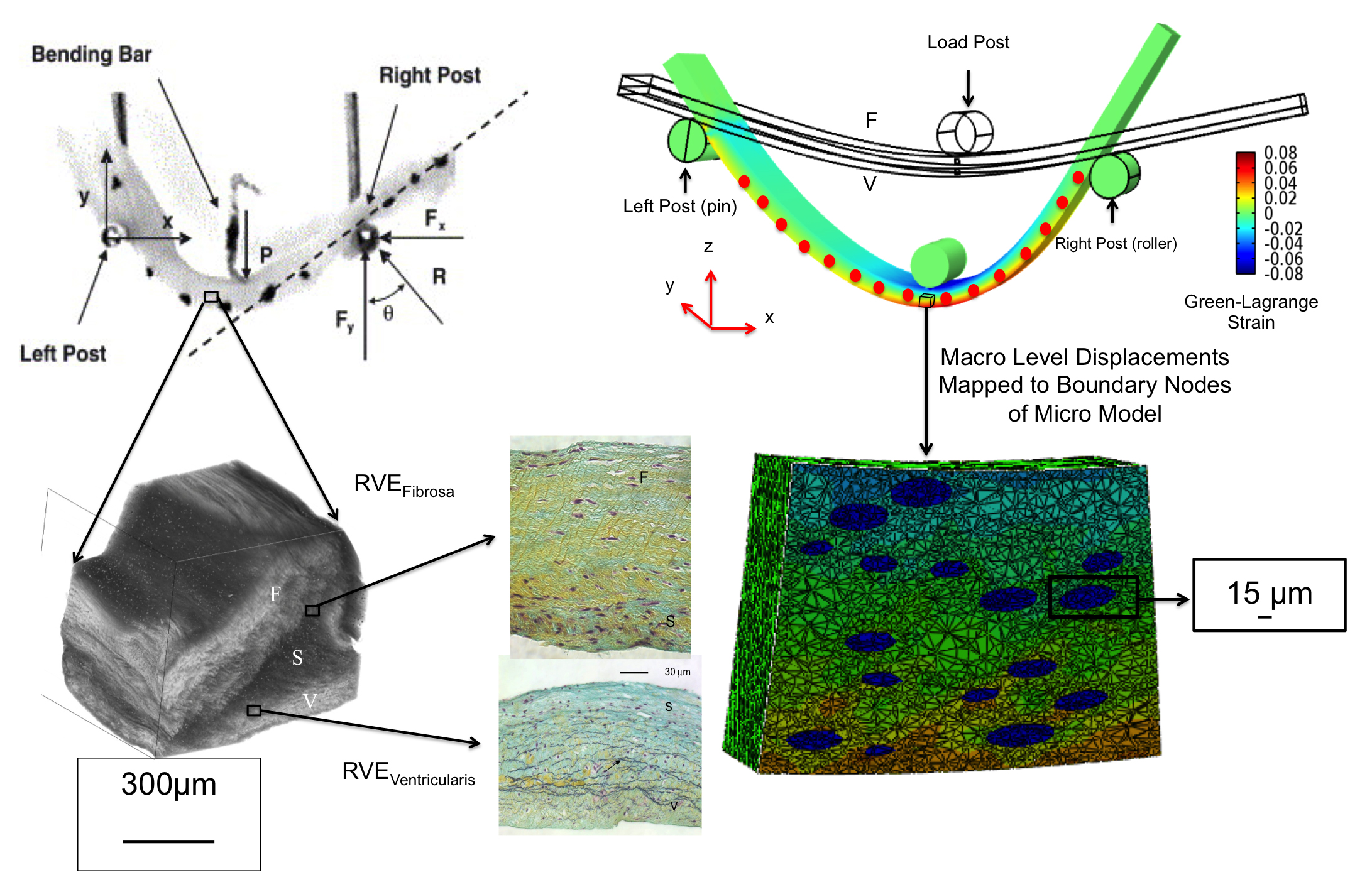
To better understand the interrelationships between tissue-level loading and cellular responses, we developed an integrated experimental-computational approach. Since in-vivo cellular deformations are not directly measurable, we quantified the in-situ layer-specific MVIC deformations for each of the four layers under a controlled biaxial tension loading device coupled to multi-photon microscopy. Next, we explored the interrelationship between the MVIC stiffness and deformation to layer-specific tissue mechanical and structural properties using a macro-micro finite element computational model.
Experimental results indicated that the MVICs in the fibrosa and ventricularis layers deformed significantly more than those in the atrialis and spongiosa layers, reaching a nucleus aspect ratio of 3.3 under an estimated maximum physiological tension of 150 N/m. The simulated MVIC moduli for the four layers were found to be all within a narrow range of 4.71-5.35 kPa, suggesting that MVIC deformation is pimarily controlled by each tissue layer's respective structure and mechanical behavior rather than the intrinsic MVIC stiffness, and that while the MVICs may be phenotypically and biomechanically similar throughout the leaflet, they experience layer-specific mechanical stimulatory inputs due to distinct extracellular matrix architecture and mechanical behaviors of the four MV leaflet tissue layers.
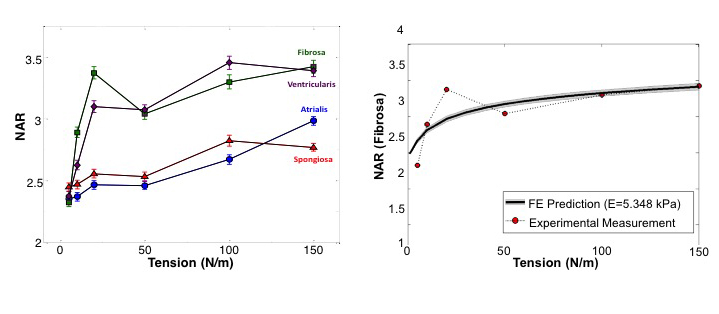
Mechanobiological Response of Valve Interstitial Cells Under Physiological Loads: Three-Dimensional Microgeometry
and Microenvironment of MVICs
Salma Ayoub
Pathophysiological alterations in mechancial loading can lead to stress-induced changes in cellular function and
tissue adaption. In the mitral valve, numerous pathological factors have been shown to affect tissue strcuture and
composition. Valve interstitial cells (VICs) are important in valve tissue homeostasis and pathophysiology: they
maintain the structural integrity of the leaflet via protein synthesis and enzymatic degradation of the
extracellular matrix (ECM). While cell phenotype and ECM regulation under physiological stress have been perviously
studied, little attention has been paid to the layer-specific structure and microenvironment of mitral VICs (MVICs).
Our aim is to fully characterize the ultrastructure of MVICs under physiological loads using serial transmission
electron microscopy and to measure and quantify the three-dimensional VIC microenvironment and geometric deformation
under physiological loads for computational model development.
Three-dimensional cell reconstruction
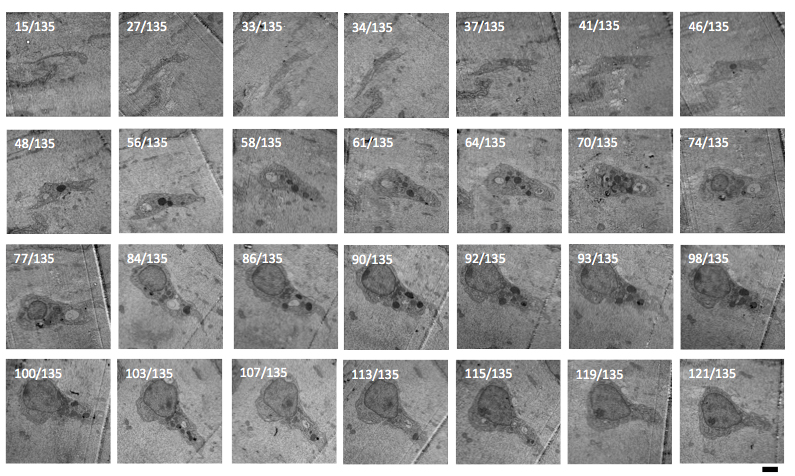
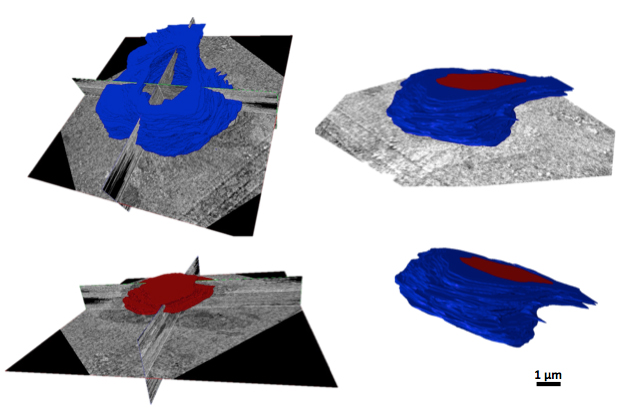
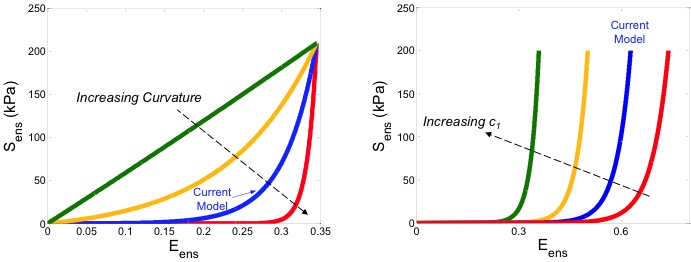
Down-Scale Modeling of Active Contraction in Aortic Valve Tissues
Rachel Buchanan
The investigation of cellular contractile behavior on tissue level stiffness in the aortic valve (AV). The tissue layers of the AV are maintained by a group of cells termed aortic valve interstitial cells (AVICs). It has been demonstrated that the contractile state of the AVICs influences the flexural stiffness properties at the tissue level. Current constitutive models exist that characterize the ECM and valve tissue behavior at the tissue level, however these models do not address the specific role of AVIC-ECM coupling contributions to the tissue-scale mechanical properties. To effectively correlate cellular level changes to the physical state of the valve, a predictive down-scale computational model has been developed. This approach provides a sensitive method to estimate AVIC and ECM mechanical properties in-situ from tissue-level experimental measurements.
Modeling Transmural Stress Variation in the Aortic Valve
Bruno Rego
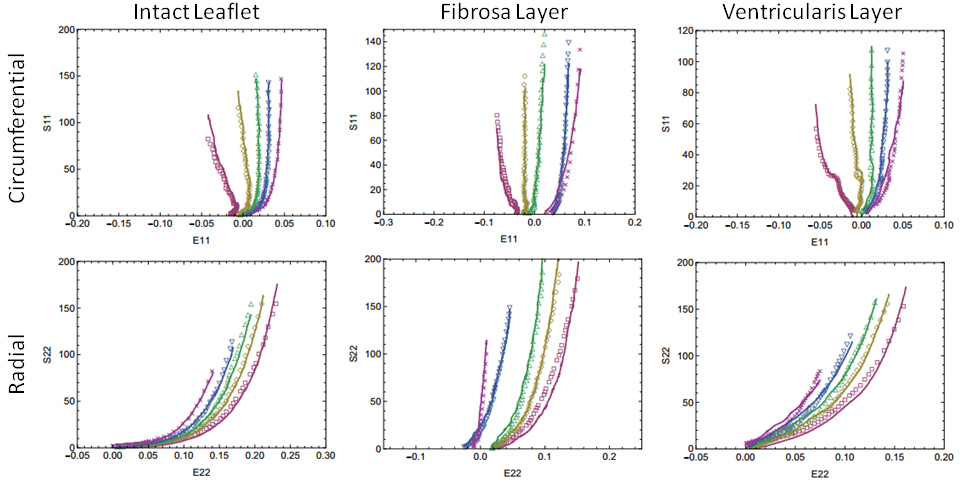
Aortic valve (AV) leaflet tissue is composed of three structurally distinct layers. While the behavior of intact AV
tissue has been thoroughly investigated, few studies have attempted to quantify the individual contributions of the
fibrosa and ventricularis layers, which account for most of the leaflet's response to external forces. By isolating
these layers via microdissection and subjecting them to various protocols of biaxial tension, our group has built an
extensive stress-strain database which illustrates the substantial layer-wise transmural variation of mechanical
properties throughout the AV leaflet (see figure). We are currently developing a theoretical framework for
integrating structural information such as fiber crimp and orientation into a constitutive model of 3D leaflet
stress, which will help uncover important structure-function relationships in the AV.
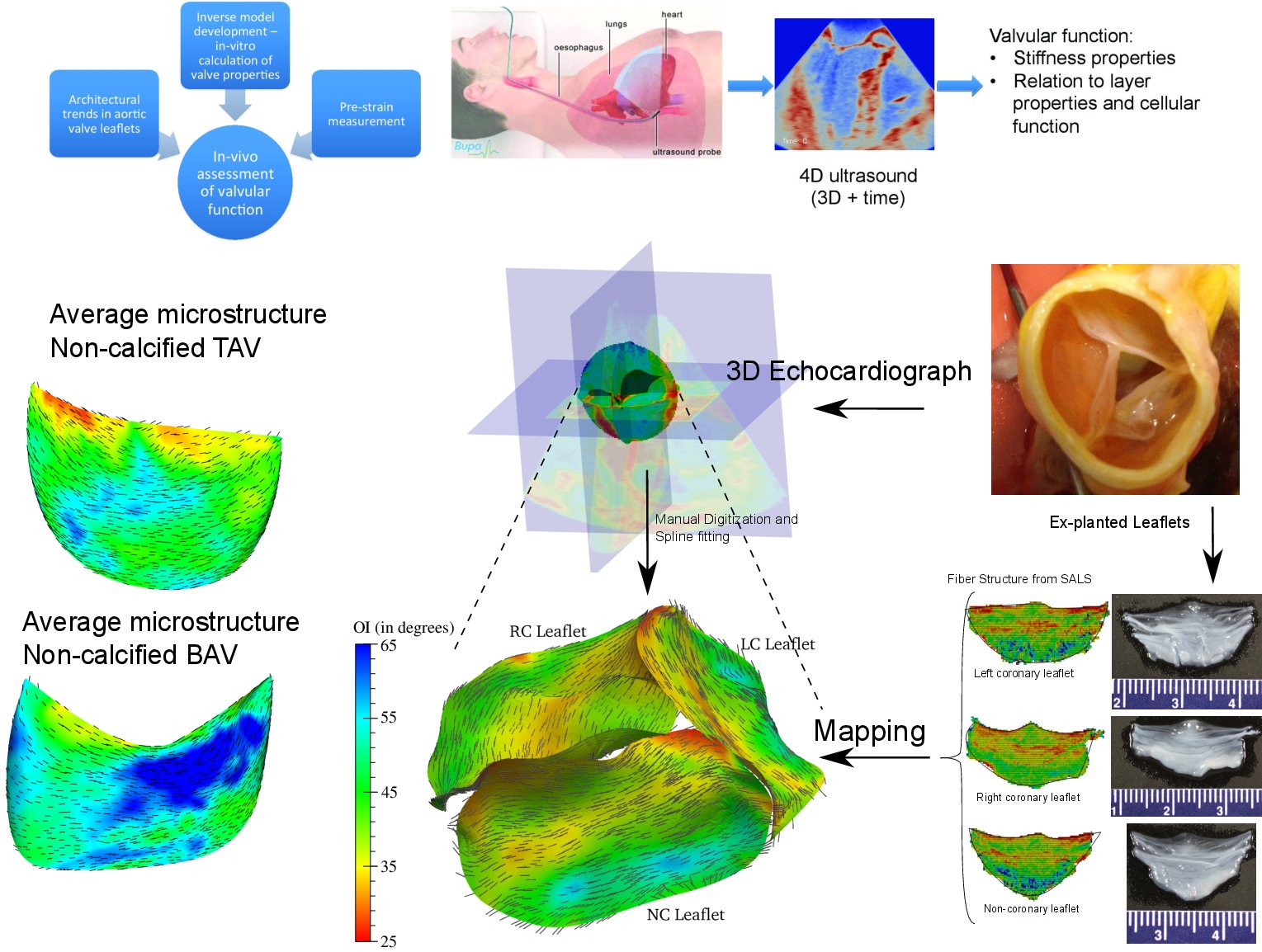
Inverse Modeling of Heart Valves with Application to Calcific Aortic Valve Disease Patient Stratification
Dr. Ankush Aggarwal
We calculate the population averaged microstructural properties of
aortic valve leaflets and use them in creating models. The aim of these
studies is to identify patients that are at a higher risk of
calcification. The microstructural differences induces interstitial
valvular cells to behave abnormally and cause the acceleration of
calcification. To identify these changes, we developed an inverse
modeling technique. The overall idea is to process the 4D ultrasound of
the patient heart and estimate the biomechanical properties of the valve
leaflets. We are applying this technique to the aortic valve from normal
and bicuspid patients. Combined with differences we observed in collagen
architecutre of two cases, we are determining the factors in leaflet
mechanics that lead to higher risk of calcific disease among patients.
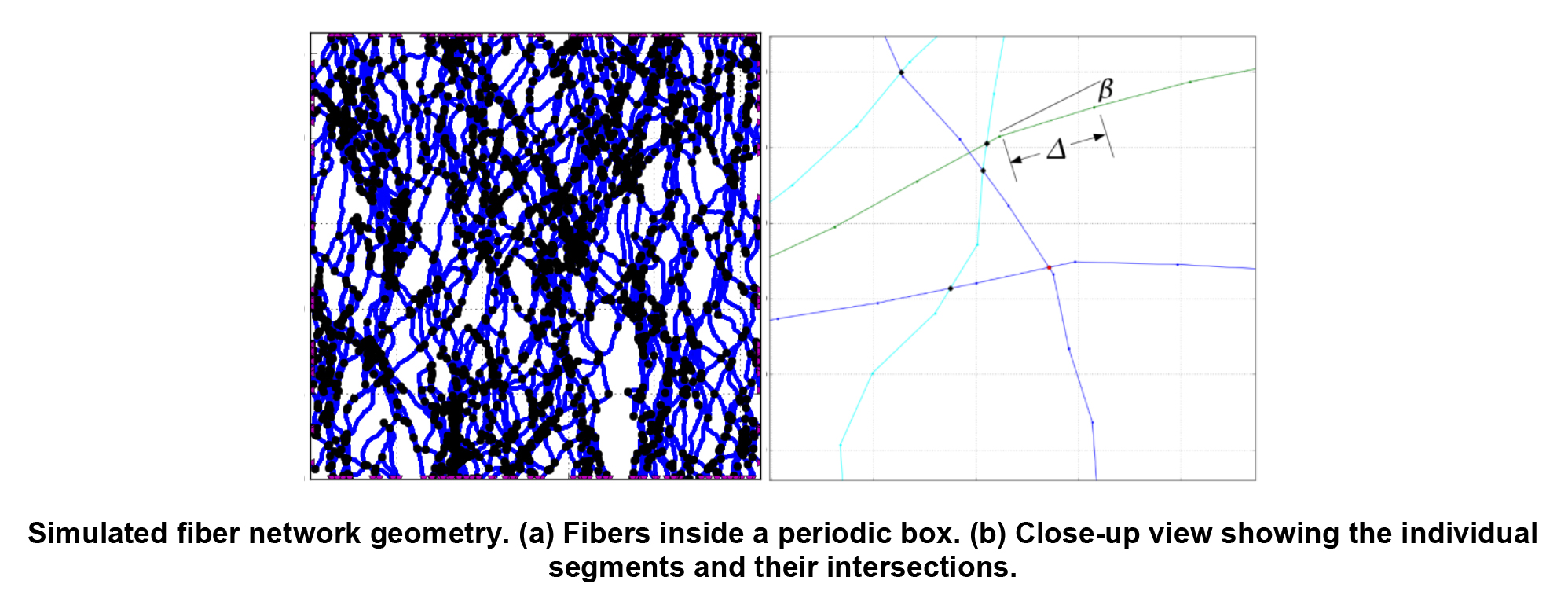
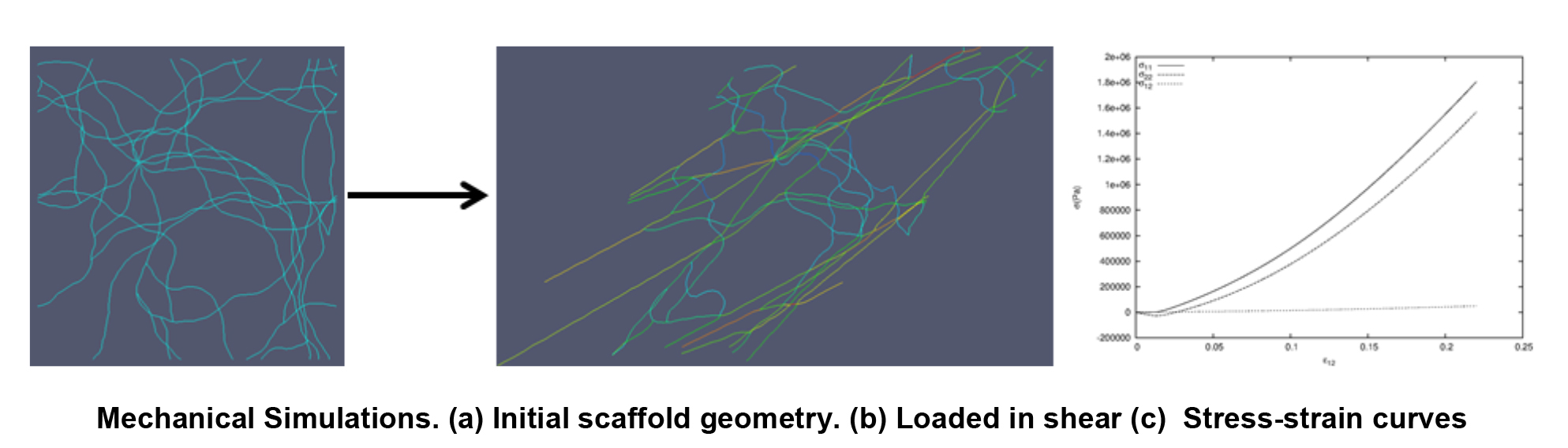
Understanding How Fiber Network Geometry of Engineered Tissue Scaffolds Affects Bulk Mechanical Behavior
Jimmy Carleton
Tissue engineers seek to produce tissues that function biologically and mechanically as well as the native tissues they are built to replace. One important step in achieving this goal is designing and manufacturing tissues that mimic the anisotropic, non-linear, large deformation mechanical behavior of the native tissues. This macroscopic behavior is strongly influenced by the evolution of the complex microstructural geometry of the underlying fibrous scaffold network. The goal of our work is to develop and use improved computational models, based on realistic fiber geometry, to help understand the mechanisms that translate scaffold fiber network structure into tissue function. We also explore the range of macroscopic material behaviors that are achievable from the domain of producible microstructural geometries and elastomeric fiber properties. Insights gained from these simulations inform macroscale material models that are essential for guiding the design of scaffolds and selecting manufacturing parameters so that the resulting engineered tissues mimic the non-linear mechanical behavior of the native tissues.
Multiscale Modeling of Myocardium
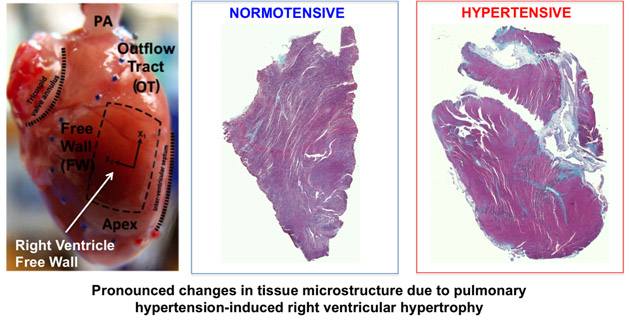
Dr. Reza Avazmohammadi
Our general interest in this project lies in understanding and modeling the mechanical behavior of the functional tissue of the heart wall, known as myocardium. In particular, we are interested in multiscale aspects of the structure-property relation in the myocardium, and in understanding how the structural properties of the myocardium at the cellular level (such as organization of myofibers and collagen network) relates to its organ-level biomechanical response. A better understanding of this relation is particularly useful in modeling phenomena such as growth and remodeling (G&R) of the hearth which involves alterations of the heart wall at the cellular level. In particular, G&R of the right ventricle, which are key predictors of right heart failure in patients with pulmonary hypertension, consist of variety of changes in the structural and mechanical properties of constituents of the myocardium and can strongly affect the organ-level functionality. Our clear understanding of how these changes correlate with the organ-level behavior can help to identify techniques for prognosis, diagnosis, and treatment of pulmonary hypertension.
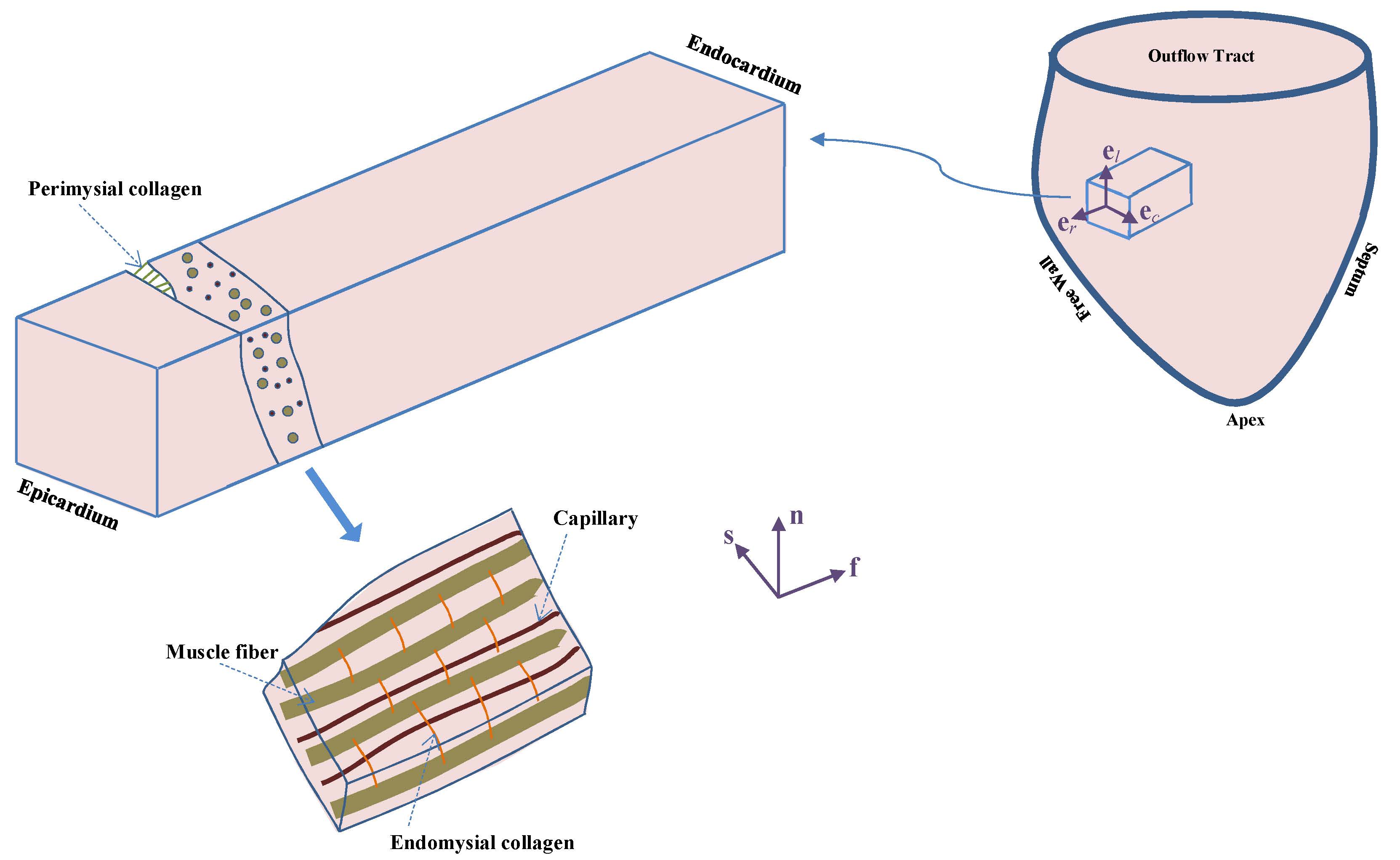
Structural Constitutive Models of Ventricular Myocardium
Dr. Michael HillDevelopment of accurate, structural constitutive models of the growth and remodeling response of right ventricular (RV) myocardium. These models will be used to predict right ventricular hypertrophy in response to pulmonary hypertension. Ultimately, clinicians may use patient-specific models to identify impending RV failure at earlier stages of this disease.
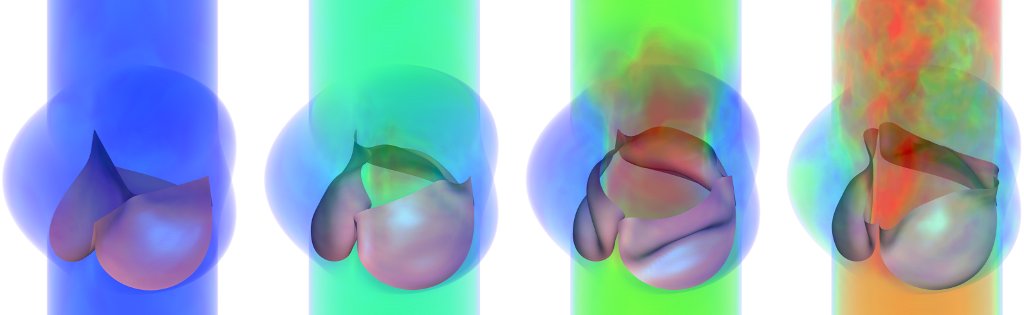
Fluid-Structure Interaction in Bioprosthetic Heart Valves
David Kamensky
Dr. Ming-Chen Hsu
This project focuses on development and application of a numerical method for fluid-structure interaction (FSI) that
is capable of simulating the mechanics of bioprosthetic heart valves operating under physiological conditions.
Valvular FSI involves large structural deformations, including changes of the fluid domain's topology as the valve
opens and closes. This leads us to pursue an approach in which a discretization of the structure moves through an
unfitted background mesh of the fluid domain. We employ the technologies of isogeometric analysis to directly
analyze spline-based representations of the valve leaflets.
Futher planned work on this project includes enhancing the accuracy and stability of the numerical method, optimizing the custom research code implementing it, realistic constitutive modeling of the valve leaflets, experimental validation, and application to problems of biomedical interest.
Bioprosthetic Heart Valve Long-Term Cyclic Loading Response Modeling
Will Zhang and Kristen FeaverDevelopment of an integrated approach to facilitate BHV biomaterial fabrication and predicting its in vivo performance under realistic stress levels in blood contact. The project is done in collaboration with Clemson University and University of Pennsylvania, utilizing novel exogenous cross-linking chemistry to preserve the native ECM components, large animal studies to examine the feasibility and time evolving biomechanics of the biomaterial under long-term cyclic loading conditions in vivo, as well as rationally developed constitutive models and rigorous computational implementations.
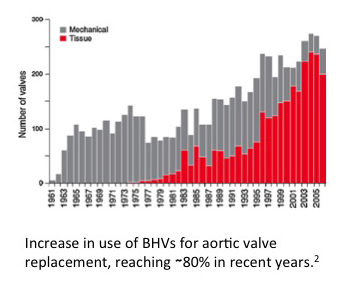
Computational Models of Dense Connective Tissue Formation for Tissue Engineered Heart Valves
Dr. Joao Soares
A myriad of external stimuli are available in current bioreactors (e.g. oscillatory flows and dynamic mechanical
conditioning) and it has become axiomatic that in vitro mechanical conditioning promotes engineered tissue
formation. However, the underlying mechanisms remain largely unknown and significant bioengineering challenges in
determining and quantifying incubation parameters that lead to optimal ECM development and structure still exist.
Efforts to date have been largely empirical, but a two-pronged approach involving novel theoretical developments and
close-looped designed experiments is necessary.
We describe cellular proliferation and ECM synthesis with a triphasic system of reaction-advection-diffusion
equations that govern the biomechanical transport and interplay of cells, ECM, and available nutrients. Effective
conditioning protocols for TE growth and development are highly dynamic and are described with FE formulations of
the evolving porous TE construct with the dynamic exterior flow resolved with CFD. Simulation results compare
favorably to existing experimental data obtained in tissue- and organ-level bioreactors, and most importantly, the
novel theoretical framework for mechanically conditioned TE growth permits the exploration/optimization of
conditioning protocols in silico in a rational and cost effective manner.
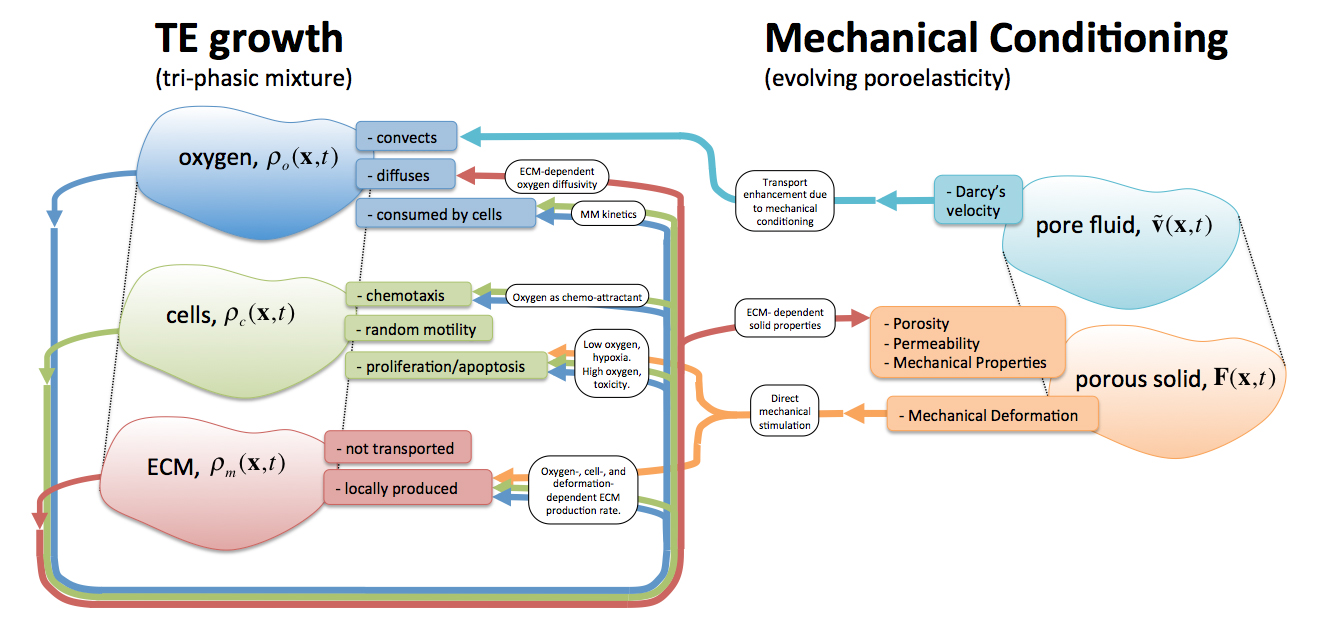
Schematic of the engineered tissue growth and development model, the ECM-evolving poroelastic material, and their two-way couplings. The tri-phasic mixture model describes the interplay between nutrient, cells, and ECM in an evolving construct. Upon mechanical conditioning, not only nutrient transport is augmented by convection through seepage velocity, but also cell proliferation and ECM synthesis are directly stimulated by the deformation.
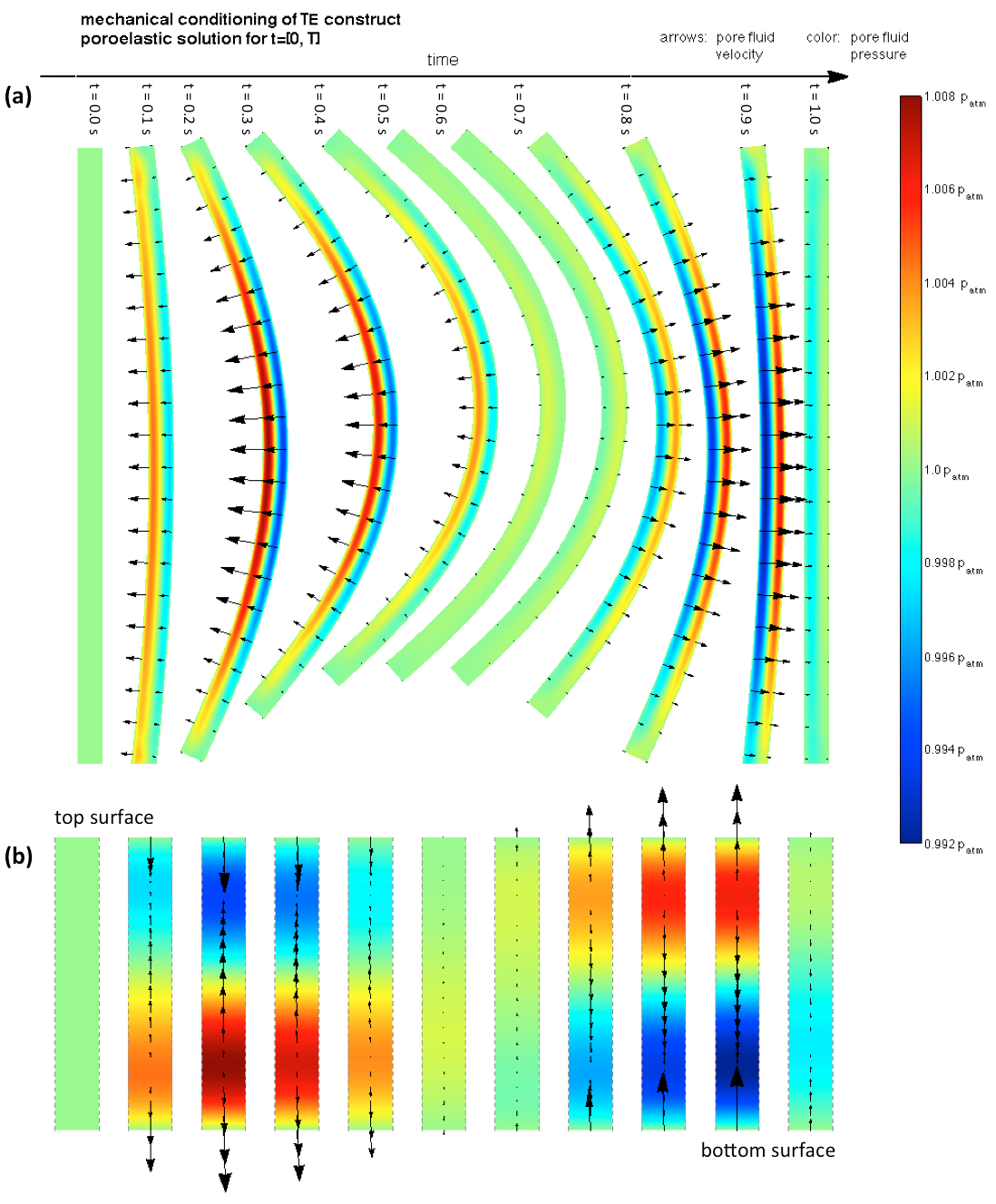
Poroelastic solution of the mechanical conditioning cycle occurring at t ϵ [0,T]. The flow field inside the construct during each cycle is substantially complex: (a) entire construct, (b) detail of the flow field occurring at the central transmural cross-section. A strong initial ejection of fluid from the bottom surface occurs when flexion initiates as this region compresses, its pore space decreases and pore pressure increases. On the other hand, fluid uptake occurs at the top surface as pore space increases. Upon deflection, reversed but approximately flow fields occur.
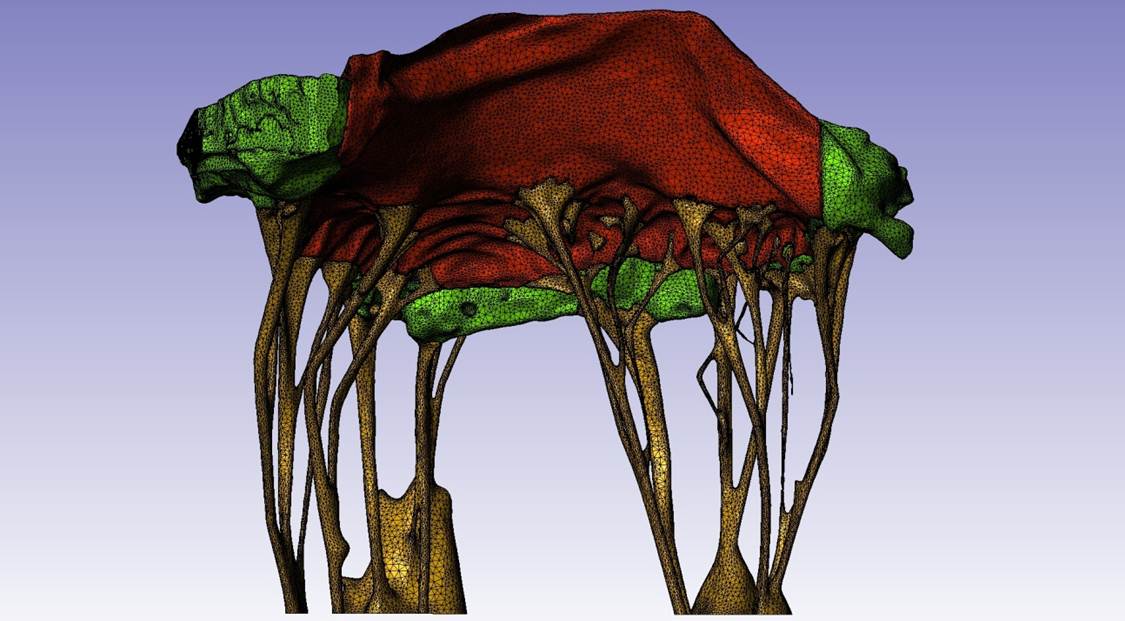
An Active Mixture Model of Valvular Interstitial Cells
Yusuke Yasamoto
Valvular interstitial cells (VICs) play a critical role in the maintenance and pathophysiology of heart valve
tissues. When activated, VICs exhibit increased levels of cytokines and extracellular matrix (ECM) snythesis and
strong contraction through the expression of α-smooth muscle actin (α-SMA) fibers. However, it remains unclear how
active contraction of the α-SMA fibers contribute to the overall VIC mechanical responses as well as other
mechanical constituents such as nucleus, cytoskeleton, and cytosolic fluid. The objective of this study is to
investigate the roles of different subcellular structures of the VICs, such as cytoskeleton, cytosolid fluid,
nucleus, α-SMA stress fibers (with different expression levels and contraction strengths) to the VIC mechanical
responses under different mechanical loading conditions and activation states. To this end, we have developed a
novel mixture model of VIC mechanics involving: 1) basal, non-oriented cytoskeletal network, 2) cytosolic fluid that
moves through the pore spaces, 3) passive elastic responses of the α-SMA stress fibers, and 4) active contraction of
the α-SMA stress fibers. The developed model integrates the data from micropipette aspiration (MA) and atomic force
microscopy (AFM) experiments, in which the VICs are under significantly different mechanical loading conditions and
activation states. Thus it enabled us to understand how the VICs function under different activation states.
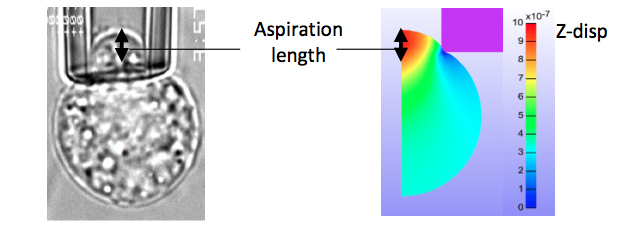
FIGURE 1: Micropipette aspiration experiment and simulation geometries.
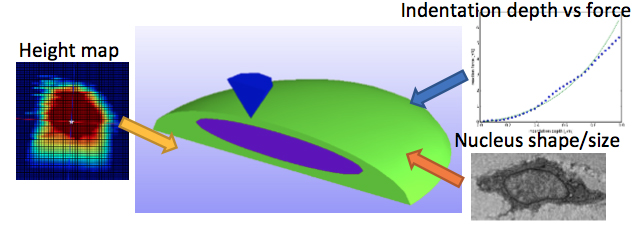
FIGURE 2: Atomic force microscopy simulation settings. The simplifed geometry was used, with cell and nucleus
dimensions taken from experimental data.
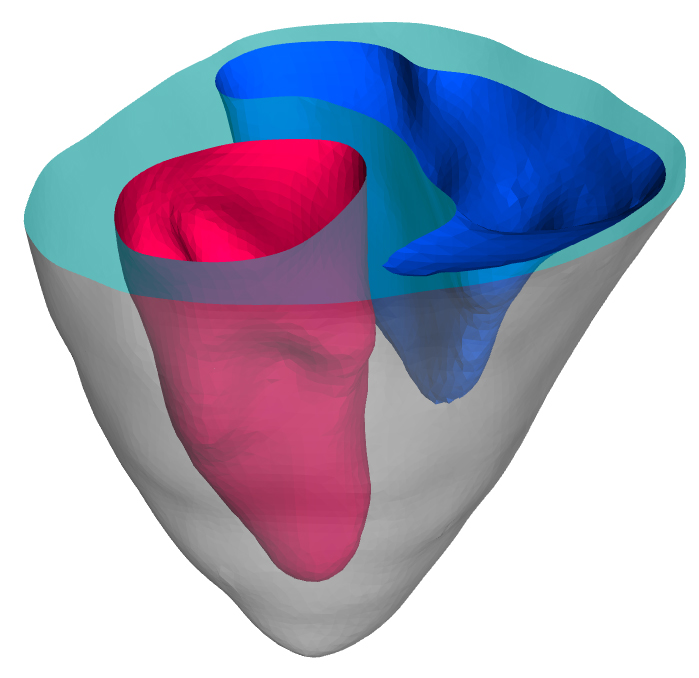
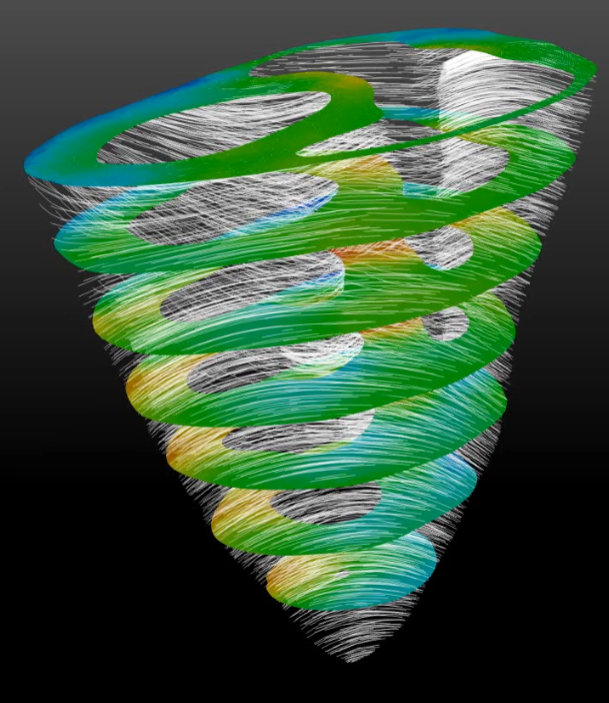
Virtual Heart Project
Dr. Samarth Raut
Virtual heart is a cardiac simulation project in collaboration with Medtronic. Computational biomechanical framework
for image based patient-specific analysis and medical device prototyping is being developed. High quality finite
element mesh of the biventricular geometry is used. User subroutines for simulating active contraction of
incompressible myocardium are developed in Fortran. Diffusion tensor MRI data is incorporated to model case-specific
spatially varying fiber architecture. In-vivo acquired pressure boundary conditions are implemented. Validation is
performed to enhance necessary confidence in the simulation results. This framework will enable exploration into
pathophysiology as well as optimal medical device design and surgical intervention.