Response of heart valve cells to deformation
Our long-term goal for this project is to understand disease processes from organ system to cellular level to develop effective treatments that mitigate early valve disease progression. Currently, there is no pharmacological treatment for any valve disease. We hypothesize that this disparity in treatment may be due to the micromechanical environment within valves greatly affecting cellular signaling outcomes of the major cell type within heart valves, valve interstitial cells (VICs).
We have chosen to first focus on understanding aortic stenosis (AS) because early progression of this disease occurs at a high rate within patients with the congenital heart defect of bicuspid aortic valve (BAV). In BAVs, in correlation with altered deformation patterns, the extracellular matrix (ECM) composition and alignment are amended, especially in the raphe region, a malformed commissure that develops between two fused leaflets of the BAV.
It has also been shown that increased VIC biaxial stretch deformation is linked to VIC activation into myofibroblasts as measured by increases in α-smooth muscle actin (αSMA). Moreover, myofibroblasts are the anticipated therapeutic target in all fibrotic conditions. In addition to mechanical cues, transforming growth factor beta (TGFβ) is implicated in VIC activation in AS.
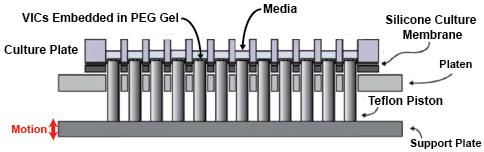
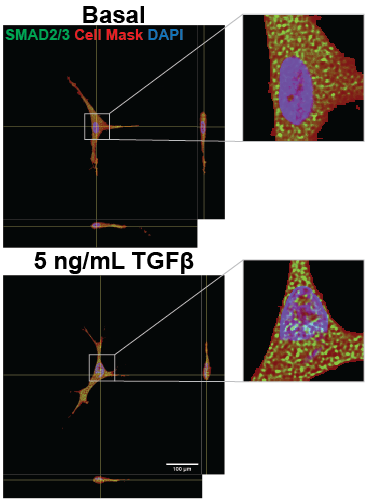
We have developed a method for encapsulating VICs in a 3D poly (ethylene) glycol (PEG) gel and conditioning the samples to 48 hours of 1 Hz waveforms of biaxial stretch using the high-throughput biaxial oscillatory stretch system (HT-BOSS). These samples can also be exposed to TGFβ and possible therapeutics to explore signaling networks and the efficacy of possible treatments. Our analysis of cytoskeletal activation, through αSMA and vimentin patterning, and transcription factor nuclear localization, such as with SMAD 2/3 nuclear localization, is enabling us to determine how biaxial stretch patterns affect VIC activation and signaling.